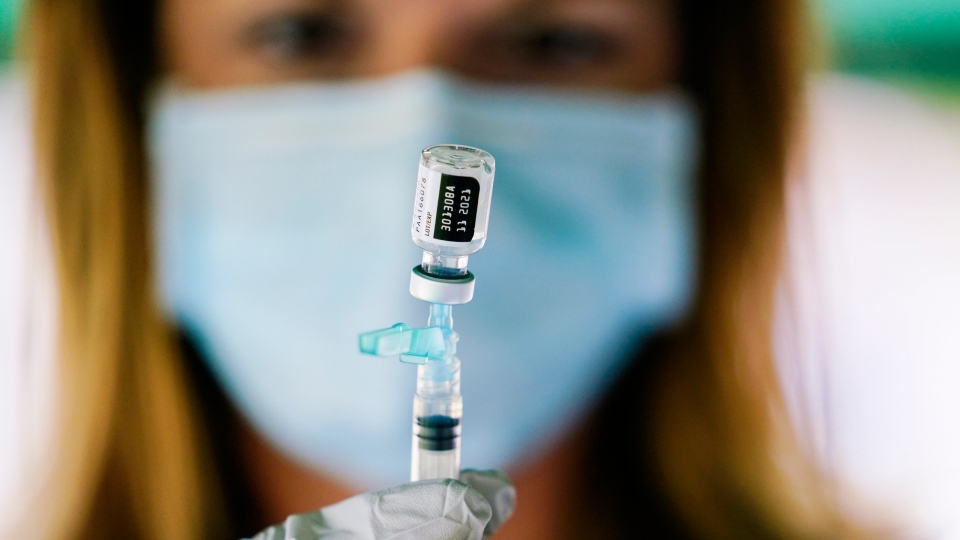
Food and Drug Administration FDA is adding a warning to the fact sheets for the PfizerBioNTech and Moderna mRNA COVID-19 vaccines as medical experts continue to. The regulatory and clinical details of this booster plan are contingent on FDA conducting an independent evaluation and determination of the safety and effectiveness of a third dose of the mRNA vaccines Pfizer and Moderna and CDCs Advisory Committee on Immunization Practices ACIP issuing recommendations based on a thorough review of the.
June 24 2021 -- The US.

Fda booster healthcare workers. For efficacy there might be a decrease as the antibody response also decreases on its own. This month lets review hepatitis B vaccination of adults. FDA advisory panel authorizes booster dose for those 65.
December 14 2020. This extra dose of a vaccine is known as a booster shot. Healthcare workers get the Pfizer-BioNTech COVID-19 vaccination at the Legacy Emanuel Medical Center on December 16 2020 in Portland Oregon.
Moderna CEO Stéphane Bancel has said people at a higher risk for severe COVID-19 including healthcare workers and older Americans could start getting booster shots by September. Along with the flu and Tdap vaccine health care workers should stay up to date on their. Occupation and healthcare setting Panlilio AL et.
Infection of vaccinated healthcare workers with the delta variant is a significant problem said study co-author Anurag Agrawal from the CSIR Institute of Genomics and Integrative Biology in Delhi. Phase 1A front-line healthcare workers and residents at long-term care facilities Texas continues to receive doses of the Pfizer Moderna and Johnson Johnson COVID-19 vaccines and is distributing statewide to hospitals pharmacies local. In a June 8 supply chain report the Biden administration the Department of Health and Human Services HHS and the Food and Drug Administration FDA called on Congress to give the FDA power to.
The frequent-risk category includes other laboratory workers such as those doing rabies diagnostic testing spelunkers veterinarians and staff and animal-control and wildlife officers in areas where rabies is epizootic. Post-exposure immunization Post-exposure efficacy of Imovax Rabies vaccine was successfully proven during clinical experience in Iran in which six 10 mL doses were given on days 0 3 7 14 30. A booster dose should be administered if the titer falls below this level.
Therefore a booster is required Eddy said. Workers in high-density worksites or worksites with large numbers of close contacts to co-workers or customers eg restaurant workers transportation workers grocery store workers Government workers with public interactions as part of their duties eg post office workers First responders eg police fire EMT and healthcare personnel. 4th Decennial Conference March 5-9 2000.
More than 350 Indonesian doctors and healthcare workers have contracted COVID-19 despite being vaccinated with Sinovac and dozens have been hospitalised officials said as concerns rise about the. Pending expected CDC and FDA approval. Ebola virus disease EVD is a very rare disease caused by infection with Zaire ebolavirus one of four types of the virus that is known to cause illness in peopleIt is believed to occur naturally in specific animal populations that live in multiple sub-Saharan African countries.
But a CDC advisory panel where the FDA made the announcement still strongly backs the vaccines benefits which outweigh the rare risk for heart inflammation. This is true even if they recently received the Td vaccine as part of the recommended vaccine schedule for all adults in which a Td booster is given every 10 years. ACIP recommends that healthcare personnel with written documentation of having received a properly spaced series of HepB in the past such as in infancy or adolescence but who now test negative for anti-HBs should receive a single booster or challenge dose of HepB and be retested 12 months later.
The latest research by the vaccine producer in China Eddy said said that a third shot is advised. Estimate of the Annual Number of Percutaneous Injuries in US. Pfizer was not.
According to the ACIP healthcare workers who havent been or are unsure if theyve been vaccinated against pertussis should get a dose of Tdap. A booster is a third dose of Sinovacs inactivated vaccine to boost the antibody against the new coronavirus. Hepatitis B vaccination recommendations vary by a persons age and risk factors.
In the Technically Speaking column in August we discussed routine hepatitis B vaccination of infants children and teens.
Us Booster Shot Campaign In Question As Fda Prepares For Key Decision Stars And Stripes

Fda Recommends Covid Vaccine Booster Shots For Ages 65 And Up People Com

Fda Advisory Panel Approves Covid Boosters For Elderly High Risk People Wkbn Com

Fda Advisory Panel Rejects Widespread Pfizer Booster Shots Wztv