Authorization EUA request to FDA for an investigational COVID-19 vaccine BNT162b2 intended to prevent COVID-19 caused by severe acute respiratory syndrome coronavirus 2 SARS-CoV-2. The FDA granted Modernas COVID-19 vaccine mRNA-1273 emergency approval for individuals aged 18 and older in December.
Roll up your.

Fda booster covid. Food and Drug Administration FDA expanded the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include adolescents 12 through 15 years of age. The Covid vaccine booster debate 23andMe hits Wall Street biotechs call of the decade By Damian Garde Meg Tirrell and Adam Feuerstein June 17 2021 Reprints. The long-term Covid-19 booster market has proven challenging for analysts to model given that it remains dependent on continuing studies about the efficacy of and necessity for booster.
The FDA officials comments come as drugmakers and some scientists now say people will likely need a booster shot of the Covid-19 vaccines. COVID booster shots study tests mixing vaccine brands. Or do the vaccines need to go through additional testing before the FDA can consider emergency use authorization.
Food and Drug Administration issued an emergency use authorization EUA for the second vaccine for the prevention of. A key panel of advisors to the CDC is set to meet Wednesday to talk about Covid vaccines including whether people may need to receive a booster. Departing FDA officials WHO leaders argue against broad rollout of COVID-19 booster shots By Ned Pagliarulo Sept.
Food and Drug Administration FDA is adding a warning to the fact sheets for the PfizerBioNTech and Moderna mRNA COVID-19. On December 18 2020 the US. The COVID-19 death toll in the US.
Executive Order 121 allows the drug regulator to issue an EUA for COVID-19 drugs and vaccines wherein an application can be submitted by the industry or government agency concerned such as the national procurer or the public health program implementer The FDA is. Topped 500000 this week and the vaccination drive has been slower than hoped. June 24 2021 -- The US.
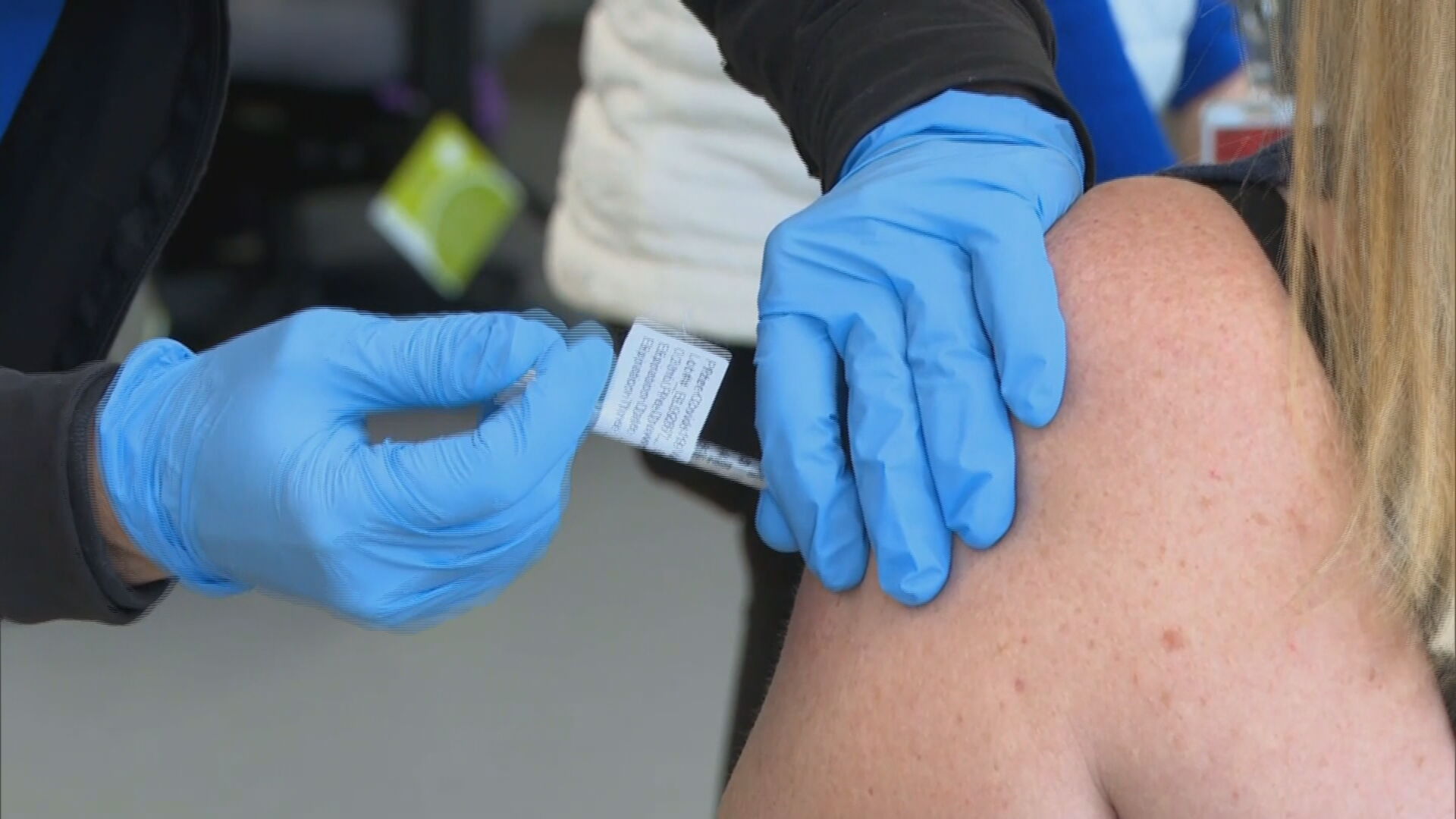
Public may need to receive a Covid-19 booster shot by the fall former Food and Drug Administration chief Scott Gottlieb said during an interview Monday. Emergency use authorization allows a vaccine to become available prior to full approval in the case of public health emergencies. Information about the Moderna COVID-19 Vaccine.
At least some portions of the US. MODERNAS COVID-19 BOOSTER VACCINE SHOWS PROMISE AGAINST VARIANTS. With that advice the FDA is expected to make a final decision within days.
11 2020 for administration in individuals 16 years of age and older. Can COVID-19 vaccine booster shots be approved after Phase 1 testing. Novavax plans to file for FDA approvals in the third quarter for its COVID-19 vaccine NVX-CoV2373 after it showed 904 overall efficacy and 93.
Pfizer says based on data coming from ISRAEL booster shots for covid are needed will ask for FDA approval. The FDA on Friday added a warning to patient and provider fact sheets for the Pfizer Inc NYSE. HealthDayA third antibody treatment designed to keep high-risk COVID-19 patients from winding up in the hospital was approved for emergency use by the US.
The FDA officials comments come as drugmakers and some scientists now say people will likely need a booster shot of the COVID-19 vaccines and possibly additional shots each year just. Food and Drug Administration on. By edhat staff Today the US.
The FDA amended the emergency use authorization originally issued on Dec. 13 2021 The sky is not falling. PFE and Moderna Inc NASDAQ.
Currently no COVID-19 vaccine is fully approved by the FDA but three - Moderna Pfizer and the currently questionable Johnson Johnson - were given emergency use authorization by the agency. Face the nation Gottlieb that sits on phizer board of director Pushes Vaccine booster on Older population.
Covid Booster Shots Fda Permits Third Dose For The Immune Deficient
Fda Experts Among Group Opposing Us Booster Shot Plan Abc News

Are Covid Boosters Needed Fda Experts Among Group Opposing Us Plan Wate 6 On Your Side

Covid Vaccine 3rd Dose Limited To Immunocompromised As World Health Organization Calls For Booster Moratorium Abc7 Chicago
Moderna Begins Submission Process For Fda Approval Of Its Covid Booster Shot Barron S

Fda Authorizes Covid Booster Shots For Certain Populations Medpage Today
Fda Sounds Skeptical Note On Pfizer Booster Shot Ahead Of Key Vote Politico

Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr
The Biden Administration May Recommend Covid Boosters After 8 Months Npr

Fda Clears Covid 19 Booster Vaccines For Some Groups

Cdc And Fda Want The White House To Slow Its Covid Booster Shot Rollout Shots Health News Npr