On Friday December 11 the FDA approved the COVID-19 vaccine from PfizerBioNTech. On May 12 2020 the US.

Fda Clears Covid 19 Booster Vaccines For Some Groups
Executive Order 121 allows the drug regulator to issue an EUA for COVID-19.
Fda approved covid vaccine booster. Approving a covid-19 vaccine now risks setting a precedent of lowered standards for future vaccine approvals. FDA granted the Moderna COVID-19 Vaccine Fast Track designation. The FDA ordered the vaccines to be tossed after determining they were not suitable for use the agency said Friday.
It was the Department of Health that applied for an EUA for the China-made vaccine. As the local Food and Drug Administration announces granting Sinopharm an emergency use authorization for its coronavirus vaccine concerns on its efficacy were raised following recent reports on its performance in other countries. On May 29 2020 the first participants in each age cohort were dosed in the Phase 2 study of the vaccine.
While the FDA and CDC say US. Anthony Fauci there could be enough information to be able to safely vaccinate children of virtually any age by the end of the year. The Food and Drug Administration has told Johnson Johnson to discard about 60 million doses of its Covid-19 vaccine that were produced at a troubled plant in Baltimore according to two people familiar with the matter.
People do not need a booster shot at this time Pfizer and BioNTech are already planning to ask for authorization to start administering COVID-19 vaccine booster. However this vaccine has not been approved to prevent or treat coronavirus or COVID-19. The Janssen vaccine is for use only in adults.
As of May 10 the Pfizer vaccine has received EUA from the FDA to vaccinate the 12-15 year old population thus making everyone 12 years old in the US. The FDA says COVID-19 vaccine recipients can donate blood. When COVID-19 vaccines were first approved by the FDA the approval was met with some skepticism from the public out of fear that the vaccines were approved too fast Johnson Johnson Pause Leads to Spike in Vaccine Hesitancy.
This vaccine may help your body develop immunity to SARS-CoV-2. And exactly one week later on December 18 the vaccine from ModernaNational Institutes of Health was given the. The FDA continues to review the results of these trials before approving or authorizing COVID-19 vaccines for use.
The side effects are more likely to affect younger people the companys research showed. Eligible for the COVID vaccine. There is no deferral time meaning vaccine recipients can donate right away if they are symptom-free and feeling well at the time.
This applies to eligible blood donors who are vaccinated with a non-replicating inactivated or RNA-based COVID-19 vaccine. Side effects from its Covid vaccine booster shots are similar to those felt after the second dose Pfizer said in new data submitted to the FDA. The FDA approved seal must represent a high barand premature licensure of a covid-19 vaccine could seriously damage public confidence in regulatory authorities particularly if long-term safety issues were to emerge following.
Pfizer is seeking approval to distribute its booster. But because there is an urgent need for COVID-19 vaccines and the FDAs vaccine approval process can take months to years the FDA first gave emergency use authorization to COVID-19 vaccines based on less data than is. The US Food and Drug Administration FDA has authorized emergency use of COVID-19 vaccine to help prevent infection with SARS-CoV-2.
Currently several COVID-19 vaccines are in clinical trials.
Moderna Begins Submission Process For Fda Approval Of Its Covid Booster Shot Barron S
Latest Fda Panel Recommends Only Limited Use Of Pfizer Boosters

What Full Fda Approval Of Pfizer S Vaccine Means For The Course Of The Pandemic
Covid Booster Shots Everything You Need To Know The Brink Boston University
Pfizer S Covid Vaccine Is More Than 90 Percent Effective In First Analysis Company Reports The Washington Post
2 Fda Officials Reportedly Resign Over Biden Administration Booster Shot Plan
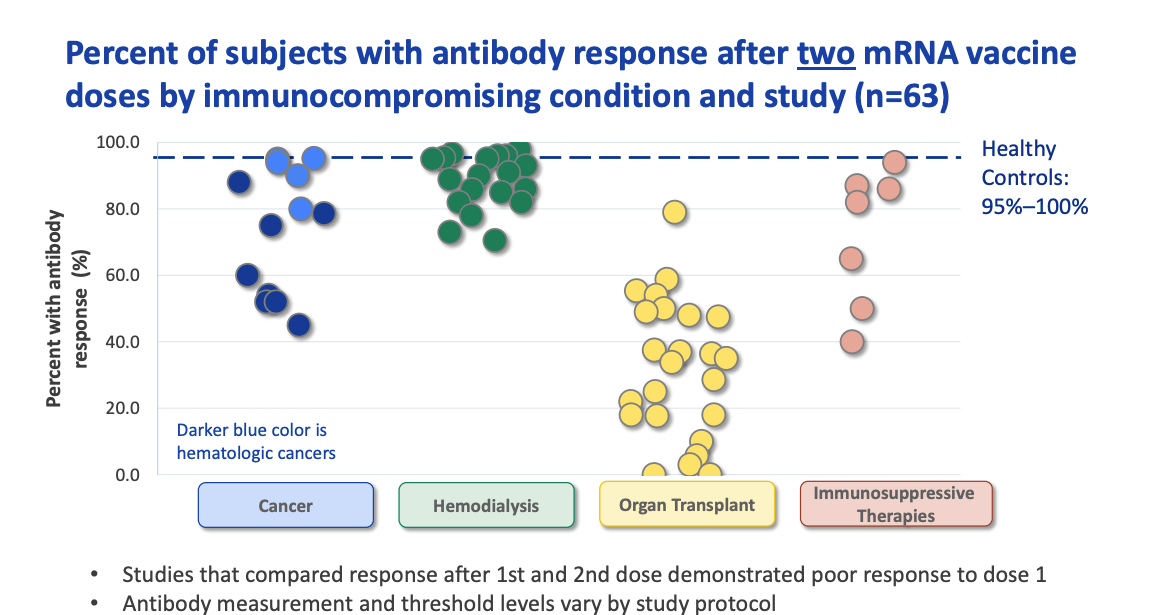
Fda Approves Covid Vaccine Booster Shots For Immunocompromised

Extra Covid 19 Vaccine Dose Ok D For Some Immunocompromised Americans Cbs News

Nj County Opens Covid 19 Booster Shot Appointments After Fda Cdc Approval
Pfizer Seeking Fda Approval For Covid 19 Vaccine Booster Dose The Times Of Israel

Covid Vaccine 3rd Dose Limited To Immunocompromised As World Health Organization Calls For Booster Moratorium Abc7 Chicago
Fda Approves Covid Vaccine Booster Shots For Immunocompromised

Covid Vaccine Moderna Applies For Full Fda Approval

Pfizer Applies For Full Fda Approval Of Boosters Side Effects And Timeline Explained Cbs 17
5 Questions About Getting Your Third Dose Of Covid 19 Vaccine Answered Houston Methodist On Health

Covid Vaccines What Full Fda Approval Means For You

Moderna Seeks Fda Approval For Booster Shots Of Its Covid 19 Vaccine Healthcare Finance News

Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr